Synthesis of bio-based non-isocyanates polyurethanes
Abstract

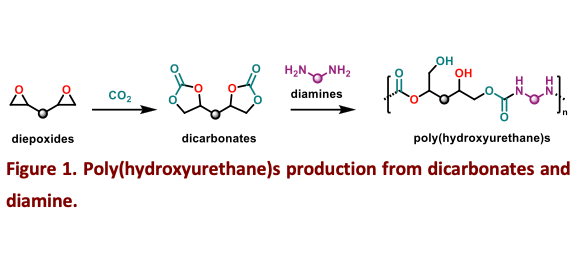
Polyurethanes (PUs) are polymers composed of hard segments (derived from isocyanates) and soft segments (derived from polyols). While PUs themselves is generally considered non-toxic, the isocyanates used in their production can pose significant health risks and contribute to environmental pollution. To improve the sustainability of polyurethane materials, non-isocyanate polyurethanes (NIPUs) are being developed. These are typically synthesized through the reaction of amines with cyclic carbonates—a process that also enables CO₂ utilization through the cycloaddition of epoxides and CO₂ to form polycyclic carbonates (Scheme 1) [1–3]. A major challenge in this field is the development of NIPUs from renewable feedstocks such as terpenes and fatty acids [4,5]. Terpenes, like limonene, can be chemically transformed into epoxides and subsequently into cyclic carbonates, offering a bio-based route to polymer synthesis [6]. Our objective is to synthesize polycyclic carbonates from terpenes and vegetable oils, followed by polyaddition reactions with diamines—including terpene-derived diamines—to obtain poly(hydroxyurethane)s. A key focus of our research will be the catalytic transformation of polyepoxides into polycyclic carbonates using organometallic complexes. This will support the optimization of polymer characteristics, including crosslinking behavior and curing conditions, to advance the sustainable production of NIPUs [7].
References
- A. Patti, F.Costa, M. Perrotti, D. Barbarino, D. Acierno Materials, 2021, 14, 1951.
- C. Pronoitis, M. Hakkarainen, K. Odelius ACS Sustain. Chem. Eng. 2022, 10, 2522.
- M. Rayung, N. A. Ghani, N. Hasanudin RSC Adv. 2024, 14, 9273.
- M. Ghasemlou, F. Daver, E. P. Ivanova, B. Adhikari Eur. Polym. J. 2019, 118, 668.
- P. Helbling, F. Hermant, M. Petit, T. Vidil, H. Cramail Macromol. Chem. Phys. 2023, 224, 2300300.
- F. Della Monica, A.W. Kleij Polym. Chem. 2020, 11, 5109.
- F. Della Monica, C. Capacchione Asian J. Org. Chem. 2022, 11, e202200300.