Stereoselective (co)polymerization of 1-vinylcyclohexene (VCH) and the (S)-4-isopropenyl-1-vinyl-1-cyclohexene (IVC) promoted by titanium complexes with [OSSO]-type ligands
Abstract
![Stereoselective (co)polymerization of 1-vinylcyclohexene (VCH) and the (S)-4-isopropenyl-1-vinyl-1-cyclohexene (IVC) promoted by titanium complexes with [OSSO]-type ligands](https://res.cloudinary.com/milan-polymer-days/image/upload/v1749635105/Screenshot_2025_06_09_alle_23_56_43_da9cf74851.png)
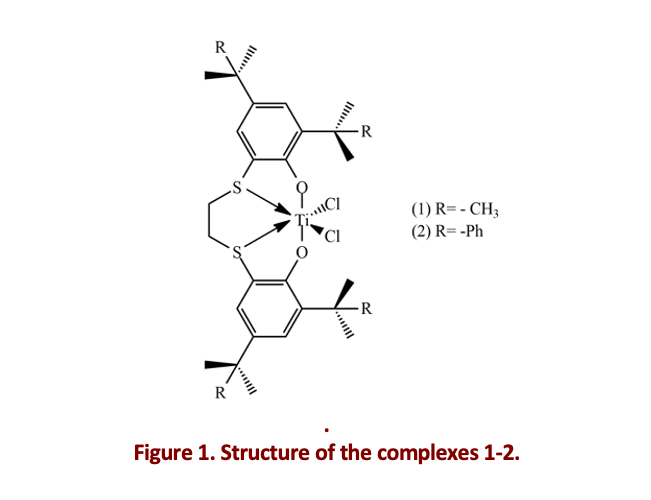
The environmental issues related to the CO2 increase in the atmosphere are pushing to new sustainable alternatives to conventional fossil resources. Thus, the production of plastics from renewable resources like carbohydrates, fatty acids, terpenes, is particularly attractive. Terpenes are abundant and inexpensive compounds, mainly present in the essential oils of flowers and plants [1]. These substances are characterized by a common isoprenoid unit; thus, they can be considered interesting starting materials for polymerization as their unsaturated structure can undergo further functionalization [2,3]. In this context, the choice of the catalysts is another challenging aspect. Metal complexes containing [OSSO]-type ligands have already proved to be effective in the polymerization of conjugated dienes, such as butadiene and isoprene [4]. In this study, the versatility and stereoselectivity of two titanium complexes with [OSSO]-type ligands (catalysts 1-2, Fig.1) is presented. First, the polymerization of the 1-vinylcyclohexene (VCH), as a model monomer, was investigated, achieving a stereo- and regio-regular homopolymer. Then, the catalysts proved to be successful in the stereospecific polymerization of the (S)-4-isopropenyl-1-vinyl-1-cyclohexene (IVC), which differs from the VCH for the isopropenyl substituent group. The IVC is a bio-based monomer synthetized from the perillaldehyde, a substance that is extracted from the essential oils of the perilla herb [5]. Furthermore, the copolymerization of VCH and IVC with styrene and two terpenes, β-myrcene and β-ocimene was accomplished, obtaining notable copolymers partially or completely from renewable sources.
References
- J. Gershenzon, N. Dudareva Nat. Chem. Biol. 2007, 3, 408.
- Y. Zhu, C. Romain, C.K. Williams Nature 2016, 540, 354.
- M. E. G. Mosquera, G. Jiménez, V. Tabernero, J. Vinueza-Vaca, C. García-Estrada, K. Kosalková, A. Sola-Landa, B. Monje, C. Acosta, R. Alonso, M. Valera Sustain. Chem. 2021, 2, 467.
- V. Paradiso, V. Capaccio, D.H. Lamparelli, C. Capacchione Coord. Chem. Rev. 2021, 429, 213644.
- H. Liu, F. You, W. Shi, X. Hu, Y. So, X. Shi Polym. Chem. 2023, 14, 4474.