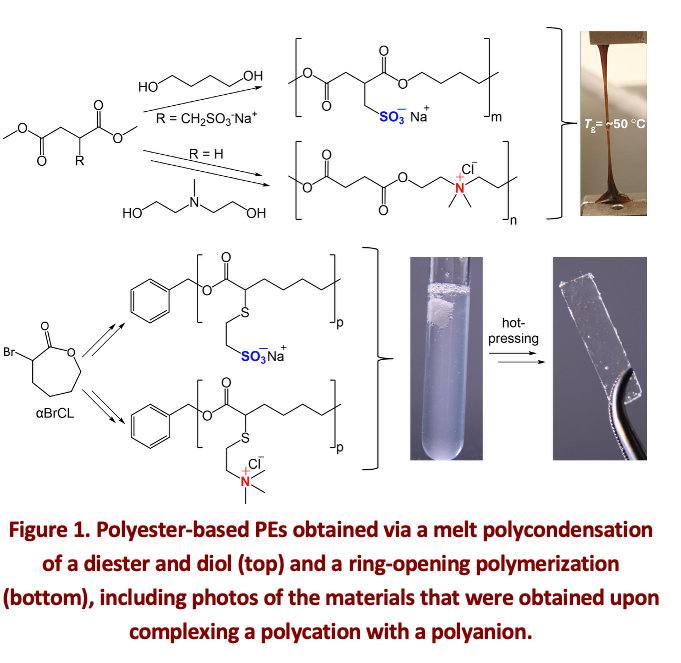
Polyester-based polyelectrolyte complexes
Abstract

Designing plastic materials with an on-demand trigger for degradation under specific environmental conditions, such as seawater, could reduce plastic pollution.[1] Polyelectrolyte complexes (PECs) made from oppositely charged polyelectrolytes are an interesting class of materials that could fulfill this requirement.[2] PECs, connected by ionic cross-links, require hydration to remain flexible but become brittle under dry conditions. The challenge in processing PECs lies in overcoming their brittleness without plasticization.
Currently, a wide range of synthetic PECs has been reported, mostly based on polymers with an all-carbon backbone. In contrast, a polyester backbone provides benefits as the ester-bond can (bio)degrade in natural environments via hydrolysis or enzymatic degradation pathways. This prompted us to design, prepare and characterize polyester-based polyelectrolytes via a melt polycondensation of diol and diester monomers (Fig. 1, top).[3] Differential scanning calorimetry (DSC) and dynamic mechanical analysis (DMA) of the resulting ester-based PEC reveal a glass-transition temperature of approximately 50 °C. Additionally, the stability of this PEC in 0.6 M NaCl was assessed, demonstrating the dissolution within 30 min, whereas PECs made from all-carbon backbones were found to be stable for > 30 days under these conditions. Furthermore, increasing the relative humidity enhances the strain at the break of the ester-based PEC. Overall, this PEC exhibits, in the dry state, a unique combination of saloplastic and thermoplastic properties.
As these first-generation polyester-based polyelectrolytes suffer from impediments such as a high dispersity and low molecular weight, discoloration, significant moisture sensitivity, and little control on charge density, we recently investigated an alternative synthetic approach that that effectively addresses these challenges. The ring-opening polymerization (ROP) of α-bromo-ε-caprolactone (αBrCL) allowed the desired precise control over dispersity and molecular weight (Fig 1, bottom). The charge density of the polyelectrolytes was controlled via post-modification of the polymer backbone with functionalized thiols for both the polyanion and polycation. The effect of charge density on the complexation behavior, the resistance against salt, and the composition of the individual polyelectrolytes in the complex were studied, aided by mean-field modelling.
Following up on the research in the liquid state, the thermal and mechanical properties of these materials in the solid state were studied, highlighting their potential for plastic products. Additionally, a comparison of the processability and mechanical properties of these second-generation materials with those of the first-generation saloplastics was made. The impact of stoichiometric versus non-stoichiometric charge density ratios on material properties—including tensile strength, thermomechanical behavior, and humidity sensitivity—is also evaluated within the context of existing plastic materials.
To conclude, while PEC materials with different charge densities were fabricated and studied in great detail, the invited presentation will largely focus on materials with a 100% charge density.
References
- G.X. Wang, D. Huang, J.-H. Ji, C. Völker, F.R. Wurm Adv. Sci. 2021, 8, 2001121.
- J. Li, S. van Lange, A. Krishna B, A. Athanasiadou, G. van Ewijk, D. J. van Dijken, J. van der Gucht, W. M. de Vos Prog. Org. Coat. 2023, 177, 107459.
- J. Engelhardt, H. Zuilhof, J. van der Gucht, L.C.P.M. de Smet, E. Maaskant ACS Appl. Polym. Mater. 2024, 6, 4409.
Acknowledgments
All the authors acknowledge financial support from the strategic investment theme ‘Connected Circularity’ of Wageningen University & Research. Louis C.P.M. de Smet gratefully acknowledges financial support from the Dutch Research Council for a Vici Grant (Mind_Gap, 19415).