Polyamidoamine-based photostabilizers for cotton fabrics
Abstract

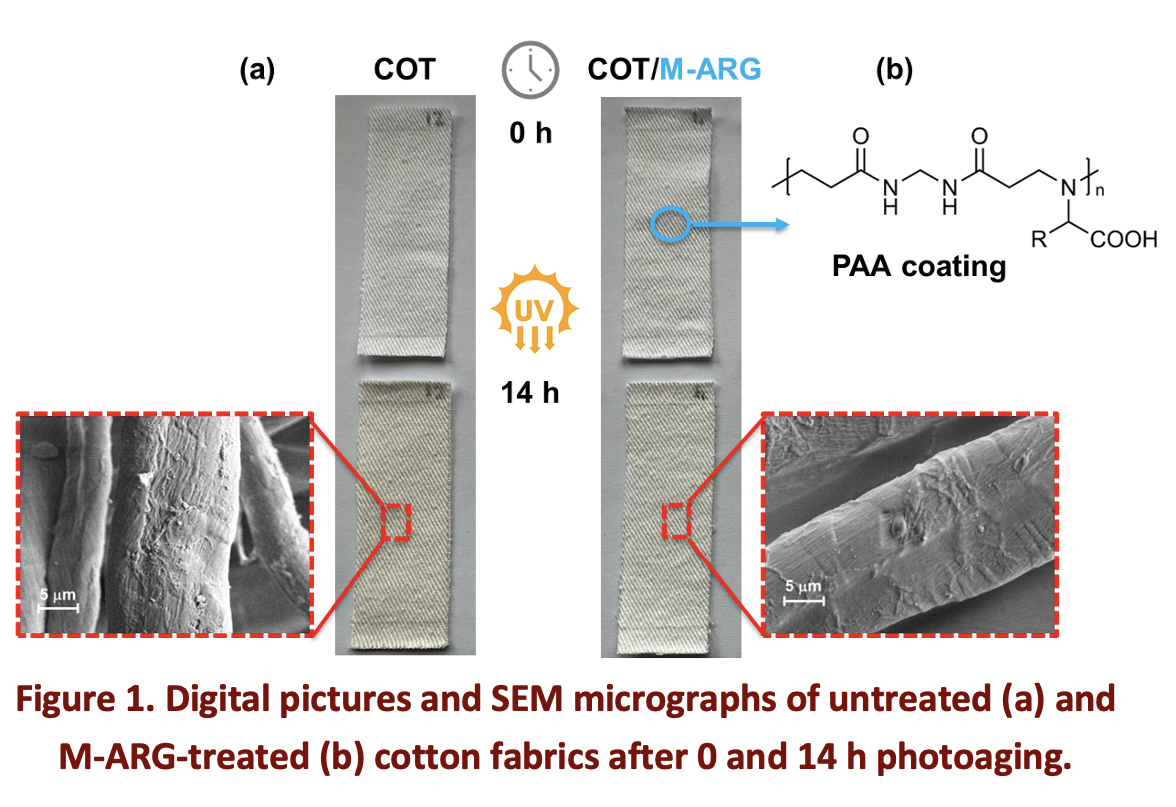
One of the major drawbacks of cellulosic materials, such as cotton, linen, and paper, is their susceptibility to photoaging when exposed to ultraviolet radiation. This process leads to discoloration, embrittlement, and the loss of aesthetic properties, which pose a significant challenge in materials science. Potential strategies to protect cellulose from photoaging include the incorporation of UV absorbers, reactive oxygen species quenchers, and antioxidants. Current commercial UV absorbers primarily include benzophenones, benzotriazoles, and triazines. These compounds are effective in mitigating UV-induced damage, but they present several limitations, particularly in terms of environmental persistence and cost-efficiency. As an alternative, polymeric photostabilizers have gathered increasing attention due to their high molecular weight, which results in lower environmental impact, reduced leaching, and an improved safety profile. Given the shared radical-based mechanisms of thermo-oxidative and photo-oxidative polymer degradation, polyamidoamines (PAAs) have been proposed as potential photostabilizers. These water-soluble biocompatible polymers, synthesized by the aza-Michael polyaddition of bisacrylamides with primary or bis-secondary amines, have already demonstrated flame-retardant properties onto cotton. Recent investigations revealed that four PAAs derived from α-amino acids effectively stabilize cotton fabrics under UVA exposure in air, attributing their effectiveness to the radical-scavenging activity of the tertiary amine groups in their polymer backbone [1]. To investigate further, a new series of PAAs was tested, bearing α-amino acid residues with different UV-absorption properties. Arginine- (M-ARG), glycine- (M-GLY), serine- (M-SER), histidine- (M-HIS) and tyrosine/arginine- (M-TYR30-ARG70) derived polyamidoamines were considered. Among them, M-HIS and M-TYR30-ARG70 absorbed radiation in the UVC and UVB wavelength range, respectively, while the others absorbed only in the far UV. Photoaging tests were performed for a total duration of 14 hours by exposing cotton strips, either untreated or with a 6% PAA add-on, to UVA and UVB irradiation in a solar chamber at 40% relative humidity and 30-35°C. Samples were analyzed using FT-IR spectroscopy and colorimetric tests at 15 min irradiation intervals evaluating CIELAB color space parameters [2]. Virgin cotton showed visible yellowing to a largely greater extent than all PAA-treated cotton (Fig. 1), except for those treated with M-TYR30-ARG70. Colorimetric analysis allowed ranking photostability of COT/PAA samples as: COT/M-ARG > COT/M-GLY > COT/M-SER > COT/M-HIS > COT > COT/M-TYR30-ARG70. Scanning electron microscopy showed slight surface erosion of untreated cotton fibers after prolonged UV-exposure, while in PAA-treated cotton, PAA coatings showed only occasional surface wrinkles and localized fractures. Following the completion of photoaging tests, PAAs were extracted from the aged PAA-treated cotton fabrics and analyzed by 1H-NMR spectroscopy. Spectral analysis revealed no significant structural changes caused by UV exposure. Overall, α-amino acid-derived PAAs protected cotton from photoaging, confirming the radical scavenging ability of tert-amine groups in the main chain. The presence of UV absorbing aromatic or heteroaromatic groups in the side chains led to worse performances due to the mobility of the α-hydrogens in radical reactions while PAAs lacking chromophores in the side chains proved intrinsically photostable. The exceptional performance of M-ARG was ascribed to its guanidine residues, engaged in electron transfer reactions with radicals, forming radical cations.
References
- J. Alongi, S. Treccani, V. Comite, P. Fermo, P. Ferruti, E. Ranucci Polym. Degrad. Stabil. 2024, 228, 110938.
- ISO 11664 Colorimetry, Part 4. 2019, International Organization for Standardization, Geneve.