Plastic degradation by wax worm enzymes: molecular insights from structural analysis
Abstract

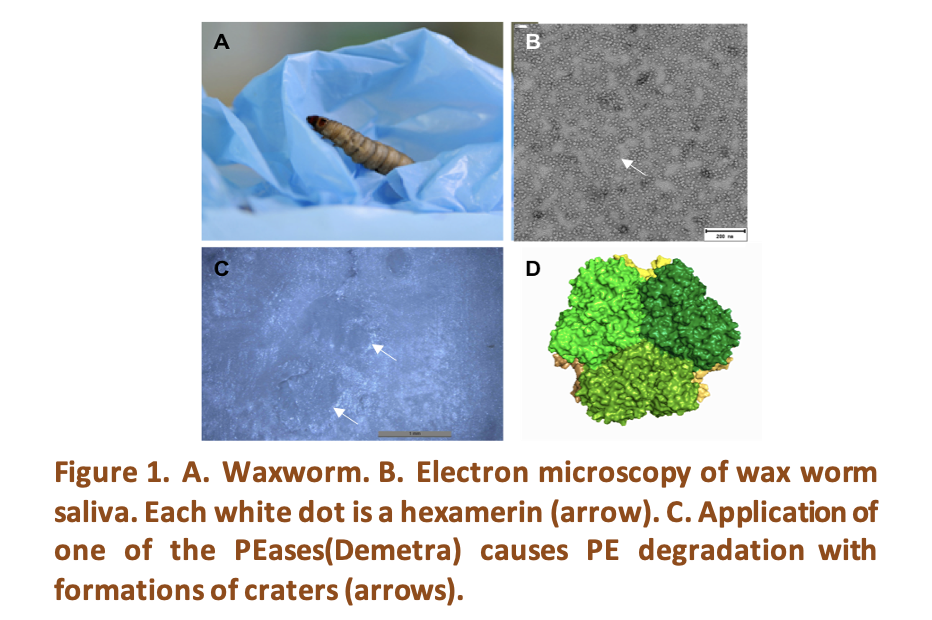
The invention of plastic materials in the 20th century marked a significant turning point in our society. Since their introduction, plastic production has continuously increased, reaching over 400 million tons per year in 2023, with projections reaching 1,200 million tons by 2060[1, 2]. However, it came with a significant drawback: plastic pollution. The remarkable durability of these synthetic materials has led to the accumulation of plastic waste in every corner of our planet. While human ingenuity succeeded in creating such an innovative material, it has yet to develop a sustainable solution for its disposal. Globally, most plastic waste ends up in landfills, with only a small percentage mechanically recycled and another portion incinerated[1, 2]. However, the effectiveness of these solutions remains highly questionable. As plastic production continues to rise, finding viable alternatives has become increasingly urgent. Recently, plastic degradation by biological means has emerged as a promising approach for tackling plastic waste. This process (bio-degradation), relies on the action of specific biocatalysts—enzymes capable of breaking down synthetic polymers. The biodegradation field is on the prowl for enzymes, looked for in microorganisms, mostly [3]. Nonetheless, this search has yielded limited success [3]. The landscape changed when the larvae of certain insects demonstrated the capacity of degrading polyethylene (PE) and polystyrene (PS) [4-6]. The first PE-degrading enzymes, termed PEases, have been discovered in the saliva of the wax worm, the larvae of the lepidopteran Galleria mellonella [7] (Fig. 1A). These PEases belong to the phenoloxidase family and are produced by the animal. Wax worm saliva is packed with four different PEases (Fig. 1B). When the saliva is applied on low density PE, oxygen is introduced into the plastic, leading to fragmentation and the formation of small oxidized compounds[7]. Recombinant PEases applied individually to PE replicate the result (Fig. 1C). Structural analyses revealed a hexameric structure (Fig. 1D), with an undefined catalytic site, but a high metal content[8]. The four hexamerins differ in the number and location of their presumed metal-binding sites[8]. We explored their degradation capacity on polyolefins other than PE, together with other synthetic polymer of interest like the polyamidoamines (PAA). Our study focuses on one of the four PEase, named Cora, the most abundant hexamerin in the worm saliva. Insights from Cryo-Electron Microscopy[8] revealed that each monomer contains four metals and a free tryptophan. A combination of classical biochemical approaches and molecular dynamics simulations will provide insights into the degradation mechanism, paving the way for optimizing the enzymatic activity and stability.
References
- https://plasticseurope.org/.
- https://ourworldindata.org/plastic-pollution.
R. Wei, W. Zimmermann Microbial. Biotechnology 2017, 10, 1308.- J. Yang, Y. Yang, W.M. Wu, J. Zhao, L. Jiang Environ. Sci. Technol. 2014, 48, 13776.
- Y. Yang, J. Yang, W.M. Wu, J. Zhao, Y. Song, L. Gao, R. Yang, L. Jiang Environ. Sci. Technol. 2015, 49, 12087.
- P. Bombelli, C.J .Howe, F. Bertocchini Curr Biol. 2017, 27, R292.
- A. Sanluis-Verdes, P. Colomer-Vidal, F. Rodriguez-Ventura, M. Bello-Villarino, M. Spinola-Amilibia, E. Ruiz-Lopez, R. Illanes-Vicioso, P. Castroviejo, R. Aiese Cigliano, M. Montoya, P. Falabella, C. Pesquera, L. Gonzalez-Legarreta, E. Arias-Palomo, M. Sola, T. Torroba, C.F. Arias, F. Bertocchini Nat Commun. 2022, 13, 5568.
- M. Spinola-Amilibia, R. Illanes-Vicioso, E. Ruiz-Lopez, P. Colomer-Vidal, F. Rodriguez-Ventura, R. Peces Perez, C.F. Arias, T. Torroba, M. Sola, E Arias-Palomo, F. Bertocchini Sci. Adv. 2023, 9, eadi6813.
Acknowledgments
The Author thanks the Italian Ministry for the University and Research, project PRIN2022 N° 202237JYZN, L’Eco-Organisme des enterprises responsables (LEKO) and (BPI) and the Banque publique d'investissement for financial support.