Non-isothermal crystallization of biobased aromatic polyesters: the role of different diacid subunits
Abstract

Aromatic polyesters cover an important class of thermoplastics used in the field of packaging thanks to their excellent mechanical properties, gas barrier, thermal stability and recyclability. Despite petroleum-based PET remaining extensively used in industrial applications, a continuously increasing number of biobased-alternatives is now available. Among these, polymers containing 2,5-furandicarboxylic acid (FDCA) and 2,5-thiophenedicarboxylic acid (TFDCA) have received significant attention due their remarkable gas barrier and mechanical properties [1,2]. However, the relationship between their molecular structure and macroscopic performance is still not fully understood. Investigation of crystallization kinetics offers a valuable mean for probing molecular features eventually useful to clarify structure-properties relations, especially for polymers containing FDCA and TFDCA, for which this topic is a key area of ongoing research.
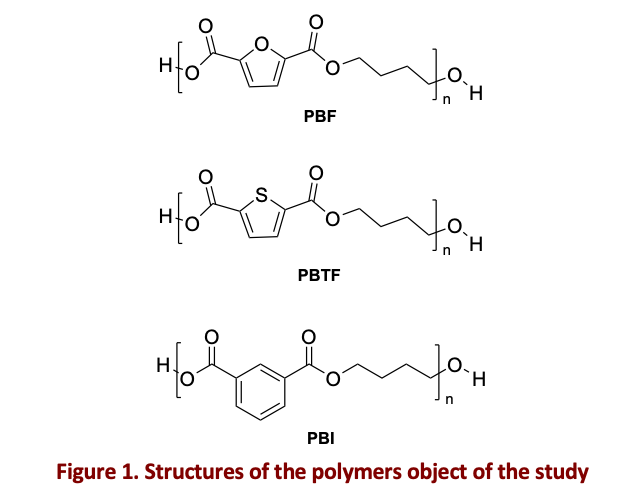
In this study, poly(butylene 2,5-furandicarboxylate)(PBF) and poly(butylene 2,5-thiophenedicarboxylate)(PBTF) were synthesized using a two-step melt polycondensation approach. Another aromatic polyester was also synthesized for the sake of comparison: poly(butylene isophthalate) (PBI). Its structure closely resembles the geometry of PBF and PBTF, making it a valuable reference for determining the role of the heteroatoms, not present in the isophthalic subunit. Non-isothermal crystallization experiments from both the melt and the glass were carried out with DSC applying different cooling and heating rates. A non-linear isoconversional method was applied to determine the dependency of the activation energy along the crystallization process [3]. The obtained effective activation energy values were fitted using the Hoffman-Lauritzen model, allowing to calculate the parameters related to nucleation and diffusion. The obtained parameters were then used to model the crystallization rate curves upon different heating/cooling rates [4], allowing to validate the calculated parameters while obtaining additional mechanistic information regarding the overall crystallization process.
The results demonstrated that different aromatic rings influenced distinct aspects of crystallization, both from the melt and from the glass, providing additional evidence to support previous hypothesis regarding the polymer microstructure. These facts highlight the applicability of crystallization kinetics study as an effective tool to investigate molecular-level properties that extend beyond crystallization related events.
References
G. Guidotti, M. Soccio, M.C. García-Gutiérrez, T. Ezquerra, V. Siracusa, E. Gutiérrez-Fernández, A. Munari, N. Lotti ACS Sustainable Chem. Eng. 2020, 8, 9558.G. Guidotti, M. Soccio, M. Gazzano, V. Siracusa, N. Lotti Polymers 2021, 13, 2460.S. Vyazovkin, N. Sbirrazzuoli J. Phys. Chem. B 2003, 107, 882.N. Guigo, J. Van Berkel, E. De Jong, N. Sbirrazzuoli Thermochimica Acta 2017, 650, 66.