Molecularly imprinted polymer for cancer imaging and therapy
Abstract

Despite undeniable progress in the prevention, screening and treatment of breast cancer, the disease remains a major health problem with alarming incidence and mortality rates worldwide. While the majority of these cancers are curable at an early stage with locoregional treatments such as surgery and/or chemotherapy-radiotherapy, the main therapeutic weapons in situations at risk of recurrence (resistance to treatment) or in metastatic situations are targeted therapies. Recently, antibody-drug conjugates (ADC) have been developed and launched to the market for this purpose [1]. However, the production of ADCs is highly complex, time consuming and costly. Thus, an alternative must be developed to provide a new approach to breast cancer treatment.
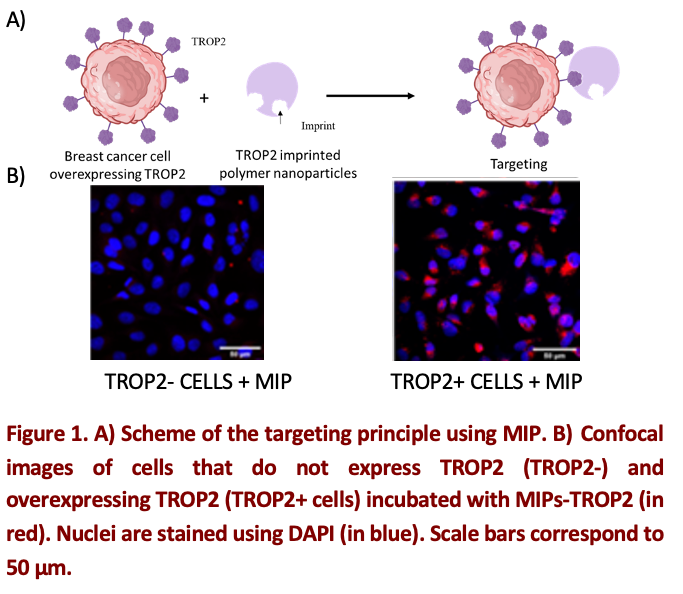
Indeed, this is what we are focusing on in our project. Using a completely new approach in the field of nanomedicine [2], we have developed fluorescent synthetic nanoparticles with molecular imprints (MIPs), encapsulating active molecules (chemotherapies, targeted therapies) used in breast cancer treatment. Moreover, as the particles are fluorescent, they can be used to detect cancer. The goal of our study is to use these MIPs nanoparticles to specifically target breast tumors overexpressing proteins (Fig. 1A) and deliver the encapsulated therapeutic molecule precisely to the tumor site.
We developed several nanomaterials able to target cells overexpressing TROP2 [3], CD44, CD36 or HER3. To achieve this, first in vitro analyses using flow cryometry, NanoITC, confocal microscopy and cell viability were performed to validate the fluorescent nanoparticles imprinting and then to assess their toxicity and biocompatibility. We used four breast cancer cell models overexpressing the protein of interest and cell models not expressing the protein as a control. Flow cytometry, confocal microscopy (Fig. 1B) and NanoITC results showed specific targeting of positive cell lines compared to the control, confirming the affinity and accessibility of the imprint to its target. In addition, no decrease in viability was observed, confirming the cellular biocompatibility of the nanoparticles. Second, in vivo studies were performed in a mouse model. The accumulation and biodistribution of the nanoparticles in the different organs of the animal were evaluated by imaging. Preliminary results show that MIPs accumulate mainly in the liver. They also accumulate slightly in the kidneys and lungs of mice. Taken together, our results confirm that the use of molecularly imprinted polymers as an alternative to ADC against cell membrane receptors represents a promising and innovative new line of nanomedicine.
Reference
- Z. Fu, S. Li, S. Han, C. Shi, Y. Zhang Sig. Transduct. Target. Ther. 2022, 7, 93.
- K. Haupt, P.X. Medina Rangel, B.T.S. Bui Chem Rev. 2020, 17, 9554.
- L. Louadj, P. Benghouzi, R. Morichon, N. Griffete, M. Sabbah ACS Appl. Nano Mater. 2025, 1, 329.
Acknowledgments
All the authors acknowledge financial support from ANR, CNRS Innovation.