Interconnected hybrid nanogel network for precision breast cancer ablation: combining multimodal imaging, sensing, and phototherapy
Abstract

Multifunctional nanomaterials for medical applications represent one of the most promising frontiers in nanoscience, particularly in the field of cancer theranostics. However, the integration of multimodal imaging and therapeutic functionalities within a single nanoplatform suitable for minimally invasive cancer treatments remains difficult[1]. Additionally, most existing tools lack the capability to detect dynamic diagnostic changes in the tumor microenvironment, such as pH fluctuations, using clinically relevant imaging modalities. These limitations hinder their translational potential and reduce the efficacy of personalized therapeutic approaches.
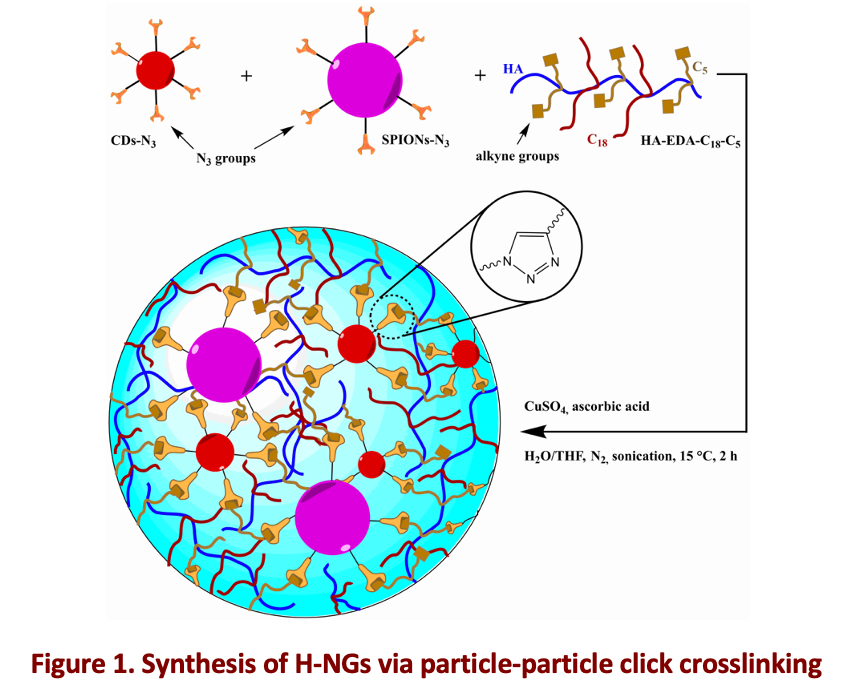
To address these challenges, we developed a hybrid nanosystem that incorporates carbon nanodots (CDs) and superparamagnetic iron oxide nanoparticles (SPIONs) within a pH-sensitive polymeric network, exhibiting pH-responsive conformational behavior (Figure1)[2,3]. SPIONs were strategically selected as auxiliary crosslinking agents due to their well-established magnetic resonance imaging (MRI) contrast properties and magnetic targeting capabilities. Simultaneously, CDs were integrated for their fluorescence and exceptional near-infrared (NIR) light conversion ability, enabling localized hyperthermia for targeted photothermal therapy under the guidance of fluorescence imaging. The resulting hybrid nanoplatforms, named H-NGs, demonstrate an enhanced ability to actively target cancer cells and effectively penetrate complex 3D multicellular cultures, which closely replicate the structural and physiological complexity of breast cancer (TNBC). Once internalized within the tumor tissue, the H-NGs facilitate real-time imaging of tumor pH and temperature variations, leveraging particle-particle distance-dependent fluorescence and MRI modulation resulting from swelling and shrinking behaviors.
Additionally, the H-NGs serve as nanoheaters, enabling precise photothermal ablation of cancerous cells under MRI guidance. This innovative nanoplatform represents a significant advancement in the development of next-generation theranostic agents. By integrating multimodal imaging, targeted therapy, and real-time TME monitoring, the H-NGs hold great potential for improving the precision and effectiveness of personalized cancer treatments. These findings pave the way for the clinical translation of smart, responsive nanomedicines, fostering new possibilities in the diagnosis and treatment of aggressive cancers such as TNBC.
References
- L. Hajba, A. Guttman, Elsevier Inc., 2016, 34, 354.
- Z. Li, S. Guo, Z. Yuan, C. Lu Sens. Actuators B Chem. 2017, 241, 821.
- N. Mauro, M. Andrea Utzeri, A. Sciortino, M. Cannas, F. Messina, G. Cavallaro, G. Giammona Chem. Eng. J. 2022, 443, 136525.
Acknowledgments
N.M. thanks Fondazione Umberto Veronesi for the support through the FUV Fellowship 2018